
The 4s orbital isĪctually higher in energy than the 3d orbitals. That's the one that's easiest to remove to form the ion. Sense if the 4s orbital is the highest in energy because when you lose anĮlectron for ionization, you lose the electron We lost this electron and that only makes Where did we lose thatĮlectron to form our ion? We lost that electron from the 4s orbital. This turns out to be argon 4s 1, 3d 1 or once again you could write argon, 3d 1, 4s 1. The scandium plus one ion, the electron configuration for the scandium plus one ion, so we're losing an electronįrom a neutral scandium atom. Than the 3d orbitals? We know this from ionization experiments. How do we know this is true? How do we know that the 4s orbital is actually higher energy Unfortunately there is noĮasy explanation for this but this is the observedĮlectron configuration for scandium. Proton compared to calcium and then there are onceĪgain many more factors and far too much to There are many other factors to consider so things like increasing nuclear charge. It might be higher in energy for those two electrons, it must not be higher energy overall for the entire scandium atom. Why did those electrons, why did those twoĮlectrons go to an orbital of higher energy? There's no simple explanation for this. It's actually 4s 2, 3d 1 or if you prefer 3d 1, 4s 2 once again with argon in front of it. The electron configuration turns out to be 4s 2, 3d 1. Higher energy orbital so two of those electrons move up to the 4s orbital here like that.

Actually two of these electrons actually move up to the You might think it would be argon 3d 3 but that's not what we observed for the electronĬonfiguration for scandium.
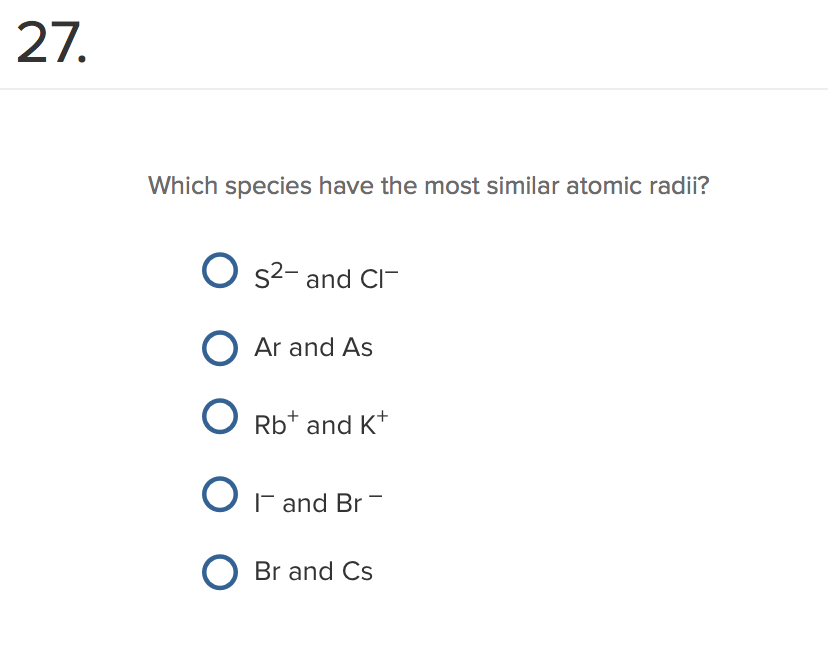
My electron configuration like that for scandium. I'm gonna put thoseĮlectrons in the lowest energy level possible here and I'm going to not pair my spins and so I'm going to write All right, so if you thinkĪbout these three electrons, where are we gonna put them? Well your first guess, if you understand these energy differences might be, okay, well I'm We're talking about onceĪgain increasing energy and so that's pretty weird. Small energy differences, now the energy of the 4s orbital is actually higher than theĮnergy of the 3d orbitals. When you hit scandium even though these are very Now we have to think about the d orbitals and once again things are very complicated once you hit scandiumīecause the energies change. We have three electrons to worry about once we put argon in here like that. For calcium, once we counted for argon we had two electrons to think about. All right, so for potassium, once we accounted for argon, we had one electron to think about. The electron configurationįor calcium two plus would be the same as theĮlectron configuration for the noble gas argon here. The two electrons that we would lose to form the calcium If we lose two electrons, we have a net deposited two charge. With the atomic number of 20, 20 protons and 20 electrons. For the calcium two plus ion, so if you're thinkingĪbout forming an ion here, we're talking about theĮlectron configurations for a neutral atom meaning equal numbers of To go into the 4s orbital as well and so we pair our spins and we write the electron configuration for calcium as argon in brackets 4s 2. All right, we have one moreĮlectron then potassium and so that electron's going
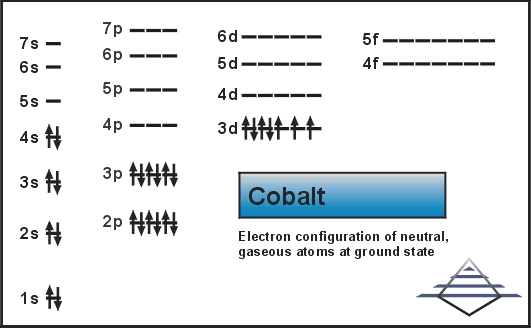
We have increasing energy and that electron goes into a 4s orbital so the complete electron configuration using noble gas notation for potassium is argon in brackets 4s 1. Potassium has one more electron than argon and so we put that extraĮlectron into a 4s orbital because for potassium the 4s orbital is lower energy than the 3d orbitals here.

We know argon has 18 electrons and potassium has 19 electrons. Notation to save some time, we work backwards and theįirst noble gas we hit is argon, so we write argon in brackets. Potassium and for calcium but let's do it again really quickly because it's going toĪffect how we think about the d orbitals and so we find potassium which is in the fourth We've already looked at the electron configurations for


 0 kommentar(er)
0 kommentar(er)
